European Chemical
News. 11-18 February 2002
Technology review: MMA know-how
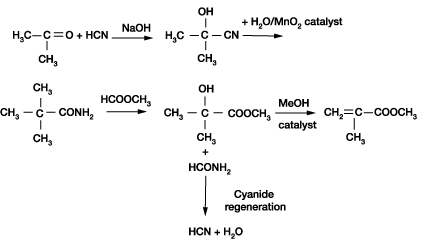
The first appreciation of possible commercial potential of acrylate and methacrylate polymers was found in Otto Roehm's doctoral thesis of 1901. Roehm formed a chemical manufacturing company with Otto Haas in 1909. Its first commercial production of methyl methacrylate (MMA) in 1933 was based on the reaction of acetone with hydrogen cyanide to form acetone cyanohydrin (ACH). This was followed by conversion of the ACH to alphahydroxy isobutyrate ester and then dehydration to the methacrylate ester using phosphorus pentachloride.
ICI published a patent in 1934 claiming a process for converting ACH to methacrylamide sulphate, which it then hydrolysed and esterified to the methacrylate ester. This is the route followed today by all modern ACH processes to MMA, accounting for roughly 80% of world total MMA capacity.
However, this process, while quite economic if a producer has access to a low-cost source of hydrogen cyanide (HCN), suffers from requiring the disposal of large amounts of ammonium bisulphate by-product. About 1.2 tonne of ammonium bisulphate is formed from every tonne of MMA produced. This disposal issue, as well as the desire to avoid using or making highly toxic HCN, has stimulated a great deal of research over the years, aimed at developing new and cost-effective process technologies. Alternative technologies have also been driven by the perceived profitability of the industry and the quest to upgrade the value of various petrochemical feedstocks available.
These research efforts have paid off and a number of alternative routes have been commercialised over the last ten to 15 years. Several other approaches are close to commercialisation. These new routes range from using new feedstocks, such as isobutylene, ethylene, or even methylacetylene (propyne) to developing techniques for recycling the HCN and/or the ammonium bisulphate. This is not to say that even within existing processes, efforts are not under way to continuously improve process performance.
The chemistries of these various approaches are outlined below, together with a discussion of competitive production costs.
At the end of 2000, there were 18 plants manufacturing MMA in three major regions, the US, western Europe and Japan, with plant sizes ranging from 30 000 tonne/year to 360 000 tonne/year. Another 11 plants also operate outside these regions.
This starts from acetone and HCN (or from purchased acetone cyanohydrin) and proceeds via dehydration, hydrolysis and esterification. This is referred to as the ACH route. Large units are based primarily on this approach. There are continued efforts to improve this process, particularly the dehydration/ hydrolysis step.
The simplified reaction chemistry is:
Mitsubishi Gas Chemical has developed a recycle version of the ACH route in which ACH is made as usual from acetone and HCN and is then hydrolysed to alpha-hydroxyisobutyramide, which is reacted with carbon monoxide and methanol under pressure to yield formamide and methyl-alphahydroxyisobutyrate.
The latter compound is dehydrated to MMA, while the co-product formamide is dehydrated to HCN for recycling. This route is referred to here as the MGC (R-HNC) route. One commercial plant is operating in Japan. The simplified reaction chemistry is as follows:
This is a two-stage gas-phase oxidation of isobutylene (or TBA) to methacrylic acid, followed by esterification. This is referred to as the i-C4 route. Such processes are operating commercially in the Far East.
A new process, in which isobutylene is first oxidised in the gas phase to methacrolein, has been developed. The methacrolein is recovered as liquid, mixed with methanol and then oxidised with air in the liquid-phase over a Pd/Pb catalyst with simultaneous esterification to MMA. This is the Asahi Chemical Direct Metha or Asahi (D) route. One commercial plant has recently started up in Japan.
The conventional two-stage gas-phase oxidation of isobutylene is very similar technically to the gas-phase oxidation of TBA. The Asahi Chemicals' Direct Metha route has only recently started operating commercially in Japan, replacing another unique process formerly used by Asahi Chemical based on methacrylonitrile.
This is the hydroformylation of ethylene to propionaldehyde, condensation with formaldehyde to methacrolein, followed by oxidation and esterification. The first company, and so far the only one, to commercialise this route is BASF and therefore it is referred to as the BASF route. One commercial plant is operating at Ludwigshafen, Germany. The process chemistry is shown in simplified form below. The process is disadvantaged by having to go through methacrolein as an intermediate because of the high cost of methacrolein oxidation.
Most new MMA plants constructed or announced in recent years have nameplate capacities in the range 35 000-60 000 tonne/ year. This scale of production is a compromise between the economies of large-scale for capital-intensive processes, versus the limitations of feasible market increments and also the limitations of raw materials supplies at each site. However, there have been examples in the US and UK of larger increments in capacity.
The patent and chemical literature contains many descriptions of research aimed at improving existing routes, or developing ingenious new routes, for the production of MMA. This brief review concerns seven of the more interesting new process routes to MMA, most of which have been, or are being, developed through the pilot plant stage and some of which have evolved to full-scale plant designs, with serious consideration having been given, or still being given, to constructing industrial facilities. Three of the processes are improved ethylene-based routes to be compared to the existing BASF ethylene-based operating plant. These three processes are:
The Ineos process, with a pilot plant under construction, relies on combined carbonylation and esterification of ethylene to methyl propionate. The methyl propionate is reacted with formaldehyde under almost anhydrous conditions to form methyl methacrylate. The process chemistry has the advantage of not involving a methacrolein intermediate.
A tentative flow-sheet* has been evolved based on ICI patents to allow for removal of water from feed formalin, recovery of unreacted formaldehyde, separation and recycling of a large stream of methyl propionate plus methanol and the purification of MMA product and of by-product propionic acid.
From economic considerations, the table below (table1) compares Nexant Chem Systems estimates of raw material costs for the conventional BASF and the Ineos processes. There is very little difference in raw material cost between the two routes. The cost competitiveness of the Alpha process is linked to lower capex and a simpler process.
Research Triangle Institute (RTI)-Eastman-Bechtel route The RTI-Eastman-Bechtel three-step process is based on hydro-carbonylation of ethylene to propionic acid, followed by condensation with formaldehyde to methacrylic acid and esterification to product MMA. An admitted fundamental problem has been the restricted life of the condensation catalysts. The published information indicates selectivity to MAA in the condensation reaction to be somewhat lower than in the Ineos process. However, overall this process potentially requires less capital investment than either the BASF or the Ineosethylene-based routes.
FOR BASF AND INEOS ALPHA Processes
| BASF process | Ineos Alpha process | ||||
|---|---|---|---|---|---|
| Units for tonne Cost/tonne, $ | Units for tonne Cost/tonne, $ | ||||
| methyl methacrylate | methyl methacrylate | ||||
| Raw materials | |||||
| Ethylene, tonne | 0.379 | 271.2 | 0.358 | 255.7 | |
| Synthesis gas, '000ft3 | 22.707 | 149.9 | 22.487 | 147.7 | |
| Methanol, tonne | 0.331 | 68.3 | 0.379 | 77.2 | |
| Formaldehyde | |||||
| (37%), tonne | 1.058 | 251.3 | 1.008 | 240.3 | |
| Catalysts/chemicals | 75.0 | 88.2` | |||
| Sub-total | 815.7 | 809.1 | |||
| By-product credits | |||||
| Acetic acid, tonne | 0.025 | 15.4 | |||
| Crude acrylic acid, tonne | 0.047 | 35.3 | |||
| Hydrogen, '000ft3 | 11.464 | 30.9 | |||
| Proprionic acid | 0.038 | 19.8 | |||
| Sub-total | 50.7 | 50.7 | |||
| Net raw materials | 765.0 | 758.4 | |||
SOURCE: NEXANT CHEM SYSTEMS
Not to be outdone, BASF has researched a route involving the simultaneous carbonylation/esterification of ethylene to methyl propionate, followed by condensation with methylal to MMA, in a simplification of the industrial BASF route it currently operates. Condensation catalyst life is unknown, but in laboratory tests the fresh catalyst displayed almost quantitative selectivity combined with high conversion, albeit with rather low turnover. An idealised version of this process, assuming a long-lasting condensation catalyst could be found, results in favorable economics for the obvious reasons of process simplicity, high conversions per pass and very high selectivity. This process has never been commercialised, probably because of inability to achieve a satisfactory life for the catalyst.
This is isobutane oxydehydrogenation to methacrolein/methacrylic acid, in an analogous process to the established isobutylene (isobutene) selective oxidation. Various process developers have worked on this route, the most advanced being Elf Atochem and Sumitomo Chemical*. The process has the attraction of lower cost raw materials. The process chemistry is summarised below.
At least three groups have published patents indicating their continued active research into the route: Elf Atochem, Sumitomo Chemical and Roehm Chemische Fabrik.
The table below (table 2) compares raw material costs for isobutane oxidation versus isobutylene oxidation for the Sumitomo route clearly demonstrating the potential for cost savings.
The process itself could be reasonably straightforward. However, even with new multicomponent catalysts based on promoted caesium and molybdenum, isobutene conversions per pass are still low (9-12%) selectivities to methacrylic acid of the order of 50%.
| Isobutene process | Isobutene process | |||
|---|---|---|---|---|
| Units for tonne Cost/tonne, $ | Units for tonne Cost/tonne, $ | |||
| methyl methacrylate | methyl methacrylate | |||
| Raw materials | ||||
| Isobutylene, tonne | 0.805 | 599.7 | ||
| Isobutane, tonne | 1.203 | 359.3 | ||
| Methanol, tonne | 0.330 | 68.3 | 0.330 | 68.3 |
| Oxygen, tonne | 2.099 | 110.2 | ||
| Catalysts/chemicals | 94.8 | 99.2` | ||
| Sub-total | 762.8 | 637.1 | ||
| By-product credits | ||||
| Acetic acid,tonne | 0.043 | 28.7 | 0.192 | 127.9 |
| Crude acrylic | ||||
| acid, tonne | 0.031 | 22.0 | ||
| Sub-total | 50.7 | 127.9 | ||
| Net raw materials | 712.1 | 509.3 | ||
SOURCE: NEXANT CHEM SYSTEMS
Carbonylation/esterification of methyl acetylene (propyne) directly to MMA, was developed in detail by Shell and the technology now belongs to INEOS. This process is very simple in concept. The main limitation is the restricted availability of the raw material. The process chemistry is summarized below:
A unique commercial route to MMA, the HCN recycle route, was developed by Mitsubishi Gas Chemical (MGC) to meet its own circumstances of restricted HCN supply and effluent restrictions. It has now developed an improved process, the MGC New Process, which recycles ammonia rather than HCN. The raw material net costs are not much improved, but there are advantages apparent in capital equipment costs and ready availability of the raw materials required.
This is the carbonylation of propylene to isobutyric acid, followed by dehydrogenation to methacrylic acid and esterification to MMA. This technology has been neglected recently despite competitive basic economics, possibly because of equipment design difficulties.
Elf Atochem and Roehm Chemische Fabrik in Germany were notably active in developing this route until about five years ago. However, propylene-based technology was never commercialised and interest in it seems to have all but disappeared.
Most of the above mentioned new routes could be designed and built within the next few years if the risks were justified by markets and business considerations. The exceptions are those processes still awaiting development of crucial catalysts, which are sufficiently rugged and long-lasting for industrial use. Other novel routes keep appearing in patents and in the literature but are usually only based on laboratory research.
The following graph (see page 23) provides a cost of production summary for MMA from a number of commercial and developing technologies. The basis for the analysis is the US Gulf Coast during quarter three 2000. The average cash cost of production for the commercial routes on the basis chosen was $1.1/kg.
On the chosen basis and comparative scale of operation, the BASF process stands out as showing the highest production cost. In addition to feedstock price and cost, the main reason for this higher cost is because the process uses propionaldehyde condensation to methacrolein, which must then be oxidised to methacrylic acid, etc. This adds complexity, significant extra capital, utilities, etc.
Improved catalysts that allow the BASF process to be modified to methyl propionate intermediate (BASF New) could offer significant cost savings to the propionaldehyde route. The INEOS Alpha process and the RTI-Eastman-Bechtel process show similar raw material costs to the BASF process, but in reality require significantly less capital investment.
Whilst the Asahi Direct oxidation route appears to show some significant reduction in raw material costs, the MGC R-HCN process economics appear to be disadvantaged by high utility costs. Although it must be said that the techno-economic description of the MGC process represents a preliminary view.
The propylene carbonylation route shows some promise, provided that oxidative dehydrogenation catalysts can improve in life and sustained activity. The propyne carbonylation/esterification route could also prove economic in the appropriate situation, provided crackers were willing to extract the feedstock. Acetylene (ethyne) is available from certain steam crackers, mainly in the US and Japan, so in principle, propyne could be obtained.
By the end of 2001 the global demand for MMA exceeded 1.9m tonne/year. Forecast average annual growth for global demand is in the 3.0-3.5% range for the short- to medium-term. While global demand is dominated by the developed economies of the US, western Europe and Japan, it is the developing economies of East Asia that will experience most rapid growth in future. Global capacity exceeds 2.4m tonne/year with new projects destined for East Asia and debottlenecks and expansions in developed economies. There may even be some capacity rationalisation, given the closure of Monacril, Huelva, Spain.
The MMA market is highly commoditised and cyclical. Hence the focus by producers to improve their technology base and raw material cost position, and build larger plants.
The Middle East continues to be a fertile ground for chemicals development and this is now not restricted to commodity polymers, but includes more specialised products. One example is the maleic anhydride/butanediol project planned for Al Jubail. Currently the Middle East produces very large quantities of MTBE for export. Continued uncertainty with the US MTBE market could lead producers to seek alternative MTBE outlets. Nexant Chem Systems has long considered the potential for an isobutylene-based chemicals complex in the Middle East that could be MTBE driven (see diagram below).
Although an MTBE to MMA route has been considered (Nipopon Shokubai), combining isobutylene-consuming projects together, e.g. butyl rubber, isoprene and MMA, could permit economies of scale to be enjoyed upstream. To make this work commercially would be a significant technical and business challenge. Under such circumstances, and were access to technology competitive, MMA in the Middle East could be a reality. In a cyclical and increasingly commodity-like market the need to reduce raw material costs, optimise capital investment and fixed costs and seek higher economies of scale will continue to make MMA R&D fertile ground for many years to come.
PEP Review 99-14
Methyl Methacrylate By Lucite's
Alpha Technology (January 2003)
http://pep.sric.sri.com/Public/Reports/Phase_99/RW99-14/RW99-14.html
Lucite (formerly Ineos Acrylics)
and Kvaerner have developed a new technology, Alpha, to produce
methyl methacrylate (MMA) from ethylene in two reaction steps.
The process is claimed to avoid the environmental problems of the
conventional acetonecyanohydrin route, which involve handling
highly toxic hydrogen cyanide and disposing of large amounts of
ammonium bisulfate by-product. In addition, the Alpha technology
uses only commodity feedstocks-ethylene is reacted with methanol
and carbon monoxide to form methyl propionate, which is then
reacted with formaldehyde to yield MMA and water.
Product selectivities in each
reaction step are 99.8% and 95%, respectively. Lucite has
recently built a fully integrated pilot plant of the process at
Teesside, England. Results have been encouraging, with an MMA
purity of 99.95% achieved since April of 2002, and an expected
lifetime of two years for the catalyst used in the second step.
The company has also announced plans to build a worldscale MMA
plant in the United States using the technology, with startup
scheduled for 2006.
In this review, we present a
technical and economic evaluation of the Alpha process, based on
the production of 250 million lb/yr (113,400 t/yr) of MMA at a
U.S. Gulf Coast location. We conclude that the total fixed
capital investment for an MMA plant using the Alpha technology is
significantly lower than that required for the same plant using
the acetone-cyanohydrin route. The estimated product value for
the Alpha process is also very competitive, despite the
relatively high energy consumption caused by low per-pass
conversion levels in the second reaction step.
アクリル板を溶かすと柔らかスポンジに 阪大教授が発見
透明なアクリル板を、アルコールと水の混合液に漬けて温めると、柔らかなスポンジに――大阪大の宇山浩教授(応用化学)が、こんな発見をした。このスポンジには微小な穴があり、ナノテクノロジー(超微細技術)の材料になるという。医療や化粧品などへの応用が期待される。
宇山さんらは他のナノ材料の研究をするうち、アクリル樹脂2〜4グラムを、水20ミリリットル、アルコール(エタノール)80ミリリットルの混合液に漬けて60度に加熱すると溶けることを発見。そのまま冷ますと、液を含んだスポンジができた。溶かす型によって自由に形を変えられる。
スポンジを調べると、約300ナノメートル(ナノは10億分の1)の粒子が連なったナノ多孔体といわれる状態になっており、弾力が生じていた。こうなる理由はよくわかっていない。ナノ多孔体は細かいものを分離・吸着する目的に使われ、DNA分析などのバイオや医療、化粧品などに使われるという。
現在、アクリル樹脂の一部は400度の熱で溶かされ、リサイクルされている。新しい手法について宇山さんは「ゼリーをつくるイメージ。従来に比べ、簡単で安価なリサイクルが可能だ。メーカー工場の廃熱なども利用できる」と話している。