IM: intramuscular ุ๗ห
SC:
subcutaneous injection ็บห
ID:
intradermal injection
็เห
IV: intraveneous รฌเห
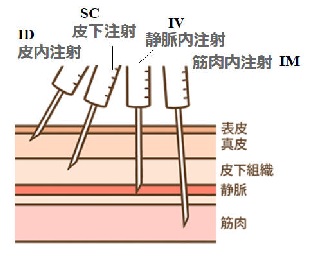
2021/2/9@WHO@The COVID-19 candidate vaccine landscape
@
| Route ห๛@
IM: intramuscular ุ๗ห |
 |
@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@๚{ฬฺํณF๊
| Developer/manufacturer | Platform | Type | doses |
Timing @days |
Route | Phase | ณF
# EUA |
@ |
| AstraZeneca + University of Oxford | Non-Replicating Viral Vector | ChAdOx1-S- (AZD1222) (Covishield) | 1-2 | 0, 28 | IM | V |
UK
2020/12/30 |
@ |
| CanSino Biological Inc./Beijing Institute of Biotechnology | Non-Replicating Viral Vector | Adenovirus Type 5 Vector | 1 | @ | IM | V |
2021/2/26 |
L๘ซ 65.28% |
| Gamaleya Research Institute ; Health Ministry of the Russian Federation | Non-Replicating Viral Vector | Gam-COVID-Vac Adeno-based (rAd26-S+rAd5-S) | 2 | 0, 21 | IM | V@ |
Sputnik V |
@ |
| Federal Budgetary Research Institution State Research Center of Virology and Biotechnology "Vector"@@@(Russia) | Protein Subunit | EpiVacCorona (EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19) | 2 | 0, 21 | IM | T/U |
EpiVacCorona |
@ |
| Janssen Pharmaceutical (Johnson & Johnson) | Non-Replicating Viral Vector | Ad26COVS1 | 1 | @ | IM | V |
FDA 2021/2/27# |
2021/5/24๚{\ฟ |
| Sinovac Research and Development | Inactivated | Inactivated | 2 | 0, 14 | IM | V |
2021/2/6 |
|
| Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products | Inactivated | Inactivated | 2 | 0, 21 | IM | V |
2021/2/26 |
L๘ซ 72.51% |
| Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products | Inactivated | Inactivated(Vero cell) | 2 | 0, 21 | IM | V@ |
2020/12/31 |
@ |
| Bharat Biotech International (Chj | Inactivated | Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) | 2 | 0, 14 | IM | V@ |
Covaxin |
@ |
| Moderna/NIAIDiฤงAM[ด๕วคj | RNA | mRNA -1273 | 2 | 0, 28 | IM | V |
FDA 2020/12/17 |
FDA 12`17@ณF๚ 2021/11/19 18ฮศใฬวมฺํฬEUA
2022/1/26 |
|
Pfizer/BioNTech
+ Fosun Pharma iใCฏใ๒j |
RNA |
BNT162 (3 LNP-mRNAs ) ปiผFCOMIRNATY |
2 | 0, 28 | IM | V |
UK 2020/12/2 |
FDA 2021/5/10
FDAณฎณFอ16ฮศใ 2021/12/9 FDA 16-17ฬวมฺํฬEUA 2022/1/3 FDA 12`15ฬวมฺํฬEUA
2022/1/25 |
| Novavaxiฤj | Protein Subunit | SARS-CoV-2 rS/Matrix M1-Adjuvant (Full length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M) | 2 | 0, 21 | IM | V |
WHO 2021/12/17 EUL |
@ |
| University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | Replicating Viral Vector | DelNS1-2019-nCoV-RBD-OPT1 (Intranasal flu-based-RBD ) | 1 | @ | IN | U | @ | @ |
| Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Protein Subunit | Recombinant SARS-CoV-2 vaccine (CHO Cell) |
2 or 3 |
0, 28 0, 28, 56 |
IM | V@ | @ | @ |
| CureVac AGiฦj | RNA | CVnCoV Vaccine | 2 | 0, 28 | IM | V | @ | @ |
| Institute of Medical Biology + Chinese Academy of Medical Sciences | Inactivated | SARS-CoV-2 vaccine (vero cells | 2 | 0, 28 | IM | V@ | @ | @ |
| Research Institute fo Bological Safety Problems, Rep. of Kazakhstan | Inactivated | QazCovid-in® - COVID-19 inactivated vaccine | 2 | 0, 21 | IM | V | @ | @ |
| Beijing Minhai Biotechnology | Inactivated | Inactivated SARS-CoV-2 vaccine (Vero cell) | 1,2 or 3 | @ | IM | U | @ | @ |
| Inovio Pharmaceuticals (ฤj+ International Vaccine Institute + Advaccine (Suzhou) Biopharmaceutical Co., Ltd | DNA | INO-4800+electroporation | 2 | 0, 28 | ID | U/V | @ | @ |
| Osaka University/ AnGes/ Takara Bio | DNA | AG0301-COVID19 | 2 | 0, 14 | IM | U/V | @ | @ |
| Zydus Cadila(Chj | DNA | nCov vaccine | 3 | 0, 28, 56 | ID | V | @ | @ |
| Genexine Consortium (ุj | DNA | DNA Vaccine (GX-19) | 2 | 0, 28 | IM | T/U | @ | @ |
| Kentucky Bioprocessingiฤj | Protein Subunit | KBP-COVID-19 (RBD-based) | 2 | 0, 21 | IM | T/U | @ | @ |
| Sanofi Pasteur/GSK | Protein Subunit | SARS-CoV-2 vaccine formulation 1 with adjuvant 1 (baculovirus production) | 2 | 0, 21 | IM | T/U | @ | @ |
| Biological E Ltd@(Chj | Protein Subunit | BECOV2 | 2 | 0, 28 | IM | T/U@ | @ | @ |
| Israel Institute for Biological Research | Replicating Viral Vector | rVSV-SARS-CoV-2-S Vaccine | 1 | @ | IM | T/U | @ | @ |
| Arcturus Therapeutics iฤj | RNA | ARCT-021 | @ | @ | IM | U | @ | @ |
| Serum Institute of India + Accelagen Pty + SpyBiotech | Virus like particle | RBD-HBsAg VLPs | 2 | 0, 28 | IM | T/U | @ | @ |
| Symvivo Corporation Ji_ | DNA based | bacTRL-Spike oral DNA vaccine | 1 | @ | oral | T | @ | @ |
| ImmunityBio, Inc & Nantkwest Inc@ฤ | Non-Replicating Viral Vector | hAd5-S-Fusion+N-ETSD vaccine | 1-2 | @ | SC or Oral | T@ | @ | @ |
| ReiTheraiษj/LEUKOCAREiฦj/UnivercellsixM[j | Non-Replicating Viral Vector | GRAd-COV2 (Replication defective Simian Adenovirus (GRAd) encoding S) | 1 | @ | IM | T | @ | @ |
| Vaxart@ฤ | Non-Replicating Viral Vector | VXA-CoV2-1 Ad5 adjuvanted Oral Vaccine platform | 2 | 0, 28 | oral | T | @ | @ |
| University of Munich (Ludwig-Maximilians) | Non-Replicating Viral Vector | MVA-SARS-2-5 | 2 | 0, 28 | IM | T | @ | @ |
| Clover Biopharmaceuticals Inc./GSK/Dynavax | SCB-2019 + AS03 or CpG 1018 adjuvant plus Alum adjuvant (Native like Trimeric subunit Spike Protein vaccine) | Native like Trimeric subunit Spike Protein vaccine | 2 | 0, 21 | IM | U/V | @ | @ |
| Vaxine Pty Ltdij | Protein Subunit | COVAX-19® Recombinant spike protein + adjuvant | 1 | @ | IM | T | @ | @ |
| University of Queensland/CSLij/Seqirusipj | Protein Subunit | MF59 adjuvanted SARS-CoV-2 Sclamp vaccine | 2 | 0, 28 | IM | T | @ | @ |
| Medigen Vaccine Biologicsiไpj/NIAID/Dynavax | Protein Subunit | MVC-COV1901 (S-2P protein + CpG 1018) | 2 | 0, 28 | IM | U | @ | @ |
| Instituto Finlay de Vacunas, Cuba | Protein Subunit | FINLAY-FR anti-SARS-CoV-2 Vaccine (RBD + adjuvant) | 2 | 0, 28 | IM | U | @ | @ |
| West China Hospital, Sichuan University | Protein Subunit | RBD (baculovirus production expressed in Sf9 cells) Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | 2 | 0, 28 | IM | U | @ | @ |
| University Hospital Tuebingen@ฦ | Protein Subunit | IMP CoVac-1 (SARS-CoV-2 HLA-DR peptides) | 1 | @ | SC | T | @ | @ |
| COVAXX + United Biomedical Inc | Protein Subunit | UB-612 (Multitope peptide based S1-RBD-protein based vaccine) | 2 | 0, 28 | IM | U/V | @ | @ |
| Merck & Co. + Themis + Sharp & Dohme + Institute Pasteur + Univeristy of Pittsburgh | Replicating Viral Vector | V591-001 - Measles-vector based (TMV-o38) | 1-2 |
0 0, 28 |
IM | T/U | @ | @ |
| Imperial College London | RNA | LNP-nCoVsaRNA | 2 | @ | IM | T | @ | @ |
| Academy of Military Science (AMS), Walvax Biotechnology i]์Xถจjand Suzhou Abogen Biosciences | RNA | SARS-CoV-2 mRNA vaccine (ARCoV) | 2 |
0, 14 or 0, 28 |
IM | T | @ | @ |
| MedicagoiJi_@cำOHป๒j | VLP | Coronavirus-Like Particle COVID-19 (CoVLP) | 2 | 0, 21 | IM | U/V |
2022/2/24@ |
@ |
| Shenzhen Geno-Immune Medical Institute | Viral vector (Replicating) + APC | Covid-19/aAPC vaccine. The Covid-19/aAPC vaccine is prepared by applying lentivirus modification with immune modulatory genes and the viral minigenes to the artificial antigen presenting cells (aAPCs). |
3 @ |
0, 14, 28 | SC | T | @ | @ |
| Shenzhen Geno-Immune Medical Institute | Viral vector (Non-replicating) + APC | LV-SMENP-DC vaccine. Dendritic cells are modified with lentivirus vectors expressing Covid-19 minigene SMENP and immune modulatory genes. CTLs are activated by LV-DC presenting Covid-19 specific antigens. | P | @ | SC & IV | T/U | @ | @ |
| Adimmune Corporation | Protein subunit | AdimrSC-2f (recombinant RBD +/- Aluminium) | @ | @ | @ | T | @ | @ |
| Entos Pharmaceuticals Inc. | DNA based | Covigenix VAX-001 - DNA vaccines + proteo-lipid vehicle (PLV) formulation |
Q @ |
0, 14 | IM | T | @ | @ |
| Providence Health & Services | DNA based | CORVax - Spike (S) Protein Plasmid DNA Vaccine | 2 | 0, 14 | ID | T | @ | @ |
| Chulalongkorn University | RNA based | ChulaCov19 mRNA vaccine | 2 | 0, 21 | IM | T | @ | @ |
| City of Hope Medical Center + National Cancer Institute | Viral vector (Non-replicating) | COH04S1 (MVA-SARS-2-S) - Modified vaccinia ankara (sMVA) platform + synthetic SARS-CoV-2 | 1-2 | 0, 28 | IM | T | @ | @ |
|
Aivita Biomedical, Inc. National Institute of Health Research and Development, Ministry of Health Republic of Indonesia |
Viral vector (Replicating) + APC | Dendritic cell vaccine AV-COVID-19. A vaccine consisting of autologous dendritic cells loaded with antigens from SARS-CoV-2, with or without GM-CSF | 1 | @ | IM | T/U | @ | @ |
| Codagenix/Serum Institute of India | Live attenuated virus | COVI-VAC |
1 2 |
0,28 |
IN | T | @ | @ |
| Center for Genetic Engineering and Biotechnology (CIGB) | Protein subunit | CIGB-669 (RBD+AgnHB) | 3 |
0,14,28 or 0,28,56 |
IM | T/U | @ | @ |
| Center for Genetic Engineering and Biotechnology (CIGB) | Protein subunit | CIGB-66 (RBD+aluminium hydroxide) | 3 |
0,14,28 or 0,28,56 |
IM | T/U | @ | @ |
| Valneva, National Institute for Health Research, United Kingdom | Inactivated Virus | VLA2001 | 2 | 0,21 | IM | T/U | @ | @ |
| Cellid Co., Ltd. | Viral vector (Replicating) | AdCLD-CoV19 (adenovirus vector) | 1 | @ | IM | T/U | @ | @ |
| GeneOne Life Science, Inc. | DNA based vaccine | GLS-5310 | 2 |
0,56 or 0,84 |
ID | T/U | @ | @ |
| Nanogen Pharmaceutical Biotechnology | Protein subunit | Recombinant Sars-CoV-2 Spike protein, Aluminum adjuvanted | 2 | 0,21 | IM | T/U | @ | @ |
| Shionogi | Protein subunit | Recombinant protein vaccine S-268019 (using Baculovirus expression vector system) | 2 | 2,21 | IM | T/U | @ | @ |
| Altimmune, Inc. | Viral vector (Non-replicating) | AdCOVID, Adenovirus-based platform expresses the receptor-binding domain (RBD) of the Sars-Cov-2 spike protein | 1-2 | @ | IN | T | @ | @ |
| University Medical Center Groningen + Akston Biosciences Inc. | Protein subunit | SARS-CoV-2-RBD-Fc fusion protein | @ | @ | SC or IM | T/U | @ | @ |
| Erciyes University | Inactivated Virus | ERUCOV-VAC, inactivated virus | 2 | 0,21 | IM | T | @ | @ |
| Vaccine and Infectious Disease Organization (VIDO) +Seppic and the Vaccine Formulation Institute (VFI) | Protein subunit | COVAC-1 and COVAC-2 sub-unit vaccine (spike protein) + SWE adjuvant | 2 | 0,28 | IM | T/U | @ | @ |